COVID-19
- Home
- COVID-19
INBIOS INDIA COVID-19
As the global health crisis caused by COVID-19 continues, InBios Indiahas partnered with various suppliers to provide quality kits for COVID-19 diagnostics. We provide kits that are US FDA, CE IVD and ICMR approved. We provide kits for different needs such as Real Time PCR kits, ELISA kits IgG or IgM, Antibody rapid tests and Antigen rapid tests. Some of our products are as below:-
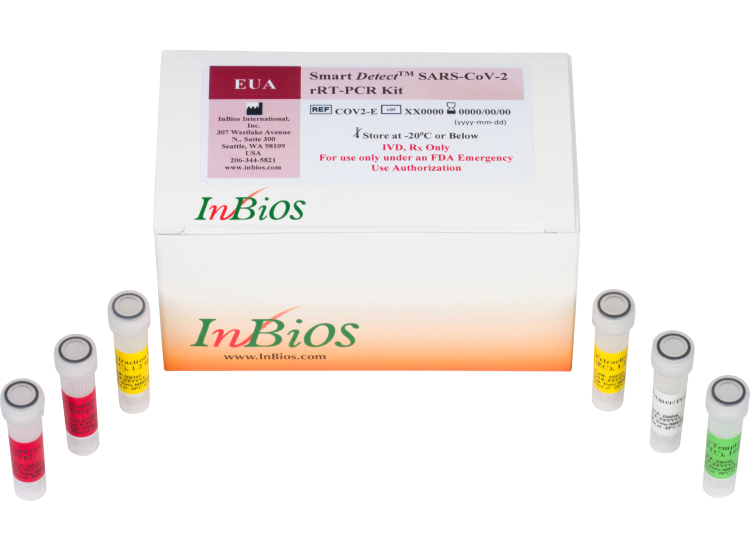
Real Time PCR Kit
Smart Detect™ SARS-CoV-2 rRT-PCR Kit
(US FDA, CE IVD and ICMR approved)
Smart DetectTM SARS-CoV-2 rRT-PCR Kit is a real-time RT-PCR test intended for the qualitative detection of nucleic acid from severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) in human nasopharyngeal swab, anterior nasal swab and mid-turbinate nasal swab specimens from individuals suspected of COVID-19 by their healthcare provider. These are US FDA, CE IVD and ICMR approved kits. These detects 3 genes E, Orf1b and N gene along with a human housekeeping gene (RNase P).
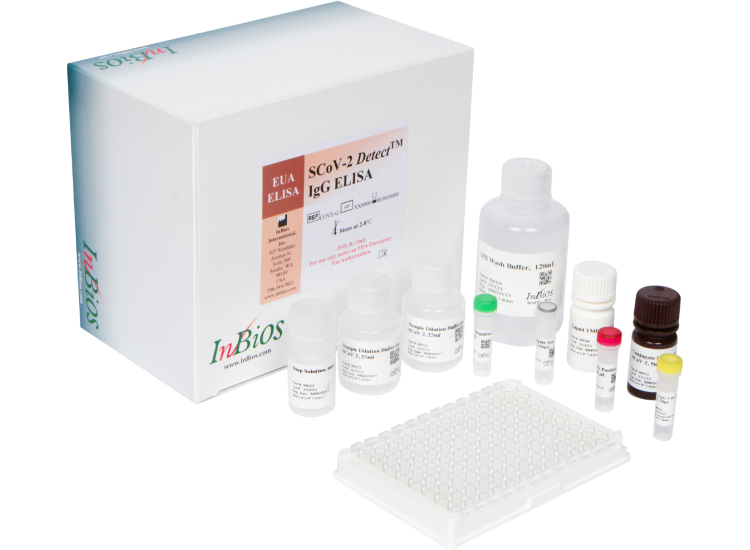
ELISA Kits
SCoV-2 Detect™ IgG ELISA
(US FDA, CE IVD and ICMR approved)
SCoV-2 DetectTM IgG ELISA kit is an in vitro diagnostic test for the qualitative detection of IgG antibodies to SARS-CoV-2 in human serum.The test is intended as an aid in identifying individuals with an adaptive immune response to SARS-CoV-2, indicating recent or prior infection. At this time, it is unknown for how long antibodies persist following infection and if the presence of antibodies confers protective immunity. The SCoV-2 Detect™ IgG ELISA should not be used to diagnose acute SARS‑CoV‑2 infection.
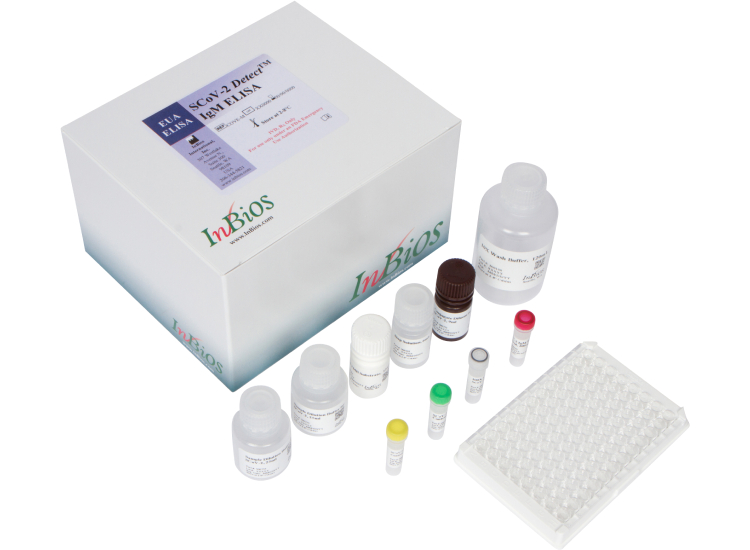
SCoV-2 Detect™ IgM ELISA
(US FDA, CE IVD and ICMR approved)
The SCoV-2 Detect™ IgM ELISA is an in vitro diagnostic test for the qualitative detection of IgM antibodies to SARS-CoV-2 in human serum.The test is intended for use as an aid in identifying individuals with an adaptive immune response to SARS-CoV-2, indicating recent or prior infection. At this time, it is unknown for how long antibodies persist following infection and if the presence of antibodies confers protective immunity. The SCoV-2 Detect™ IgM ELISA should be used to diagnose acute SARS‑CoV‑2 infection.
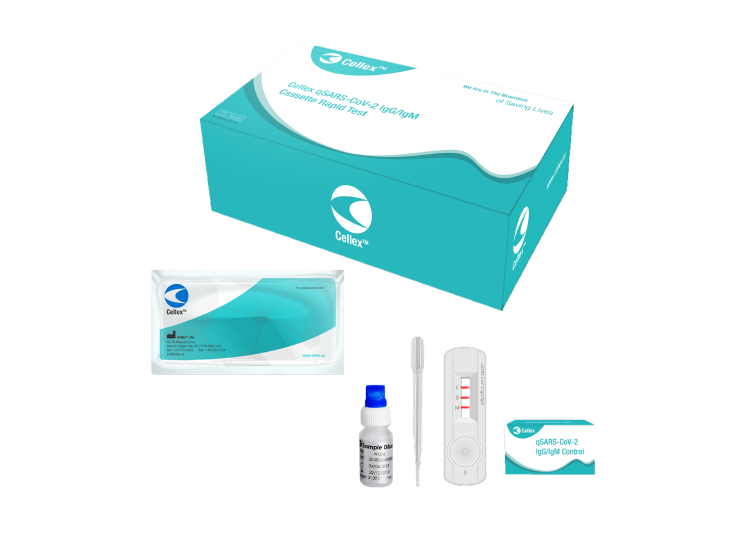
Rapid Test Kits
Cellex qSARS-CoV-2 IgG/IgM Rapid Test
(US FDA, CE IVD and ICMR approved)
The Cellex qSARS-CoV-2 IgG/IgM Rapid Test is a lateral flow immunoassay intended for the qualitative detection and differentiation of IgM and IgG antibodies to SARS-CoV-2 in serum, plasma (EDTA, citrate) or venipuncture whole blood specimens from patients suspected of COVID-19 infection by a healthcare provider. The qSARS-CoV-2 IgG/IgM Rapid Test is an aid in the diagnosis of patients with suspected SARS-CoV-2 infection in conjunction with clinical presentation and the results of other laboratory tests.
BIOPANDA COVID-19 Rapid Test Kit
BIOPANDA COVID-19 Rapid Test Kit
(MHRA-UK, CE IVD and ICMR approved)
The Biopanda COVID-19 Rapid Test Kit is a qualitative lateral flowimmunochromatographic assay for the detection of IgM and IgG antibodiesto SARS-CoV-2 in human whole blood, serum or plasma samples.It is intended as a tool for carrying out serological epidemiological investigations. It is also intended for use as a tool to assist in the diagnosisof SARS-CoV-2 infections, in conjunction with other tests.



